Summary¶
AdK secondary and tertiary structure¶
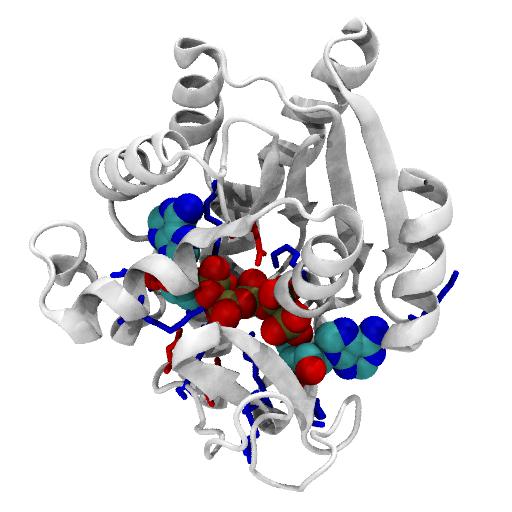
AdK is a dynamic enzyme, which can undergo a large conformational change between an open and a closed conformation. The backbone RMSD between the two conformations is about 7.16 Å.
AdK contains a central beta sheet (with strands A1 to A5) between four helices (H1, H5, H7, H8), making up the CORE domain (residues 1-29, 60-121, 160-214). This CORE domain does not change much between the open and closed conformation (RMSD 1.97 Å of CORE).
The NMP domain, consisting of helices H2—H4 (residues 30-59), and the LID domain (H6 and two small beta sheets B1/B2 and C1/C2/C3, resids 122-159) are very mobile. On closing, they both fold towards CORE on hinges. When RMS-fitted on CORE, the overall RMSD is 8.0 Å.
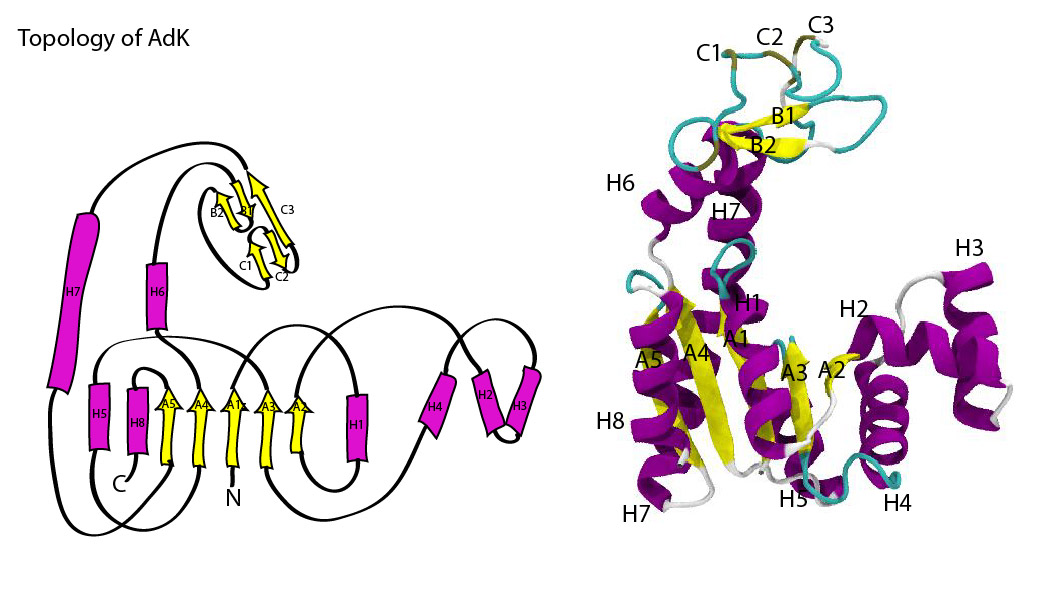
Topology diagram of AdK.
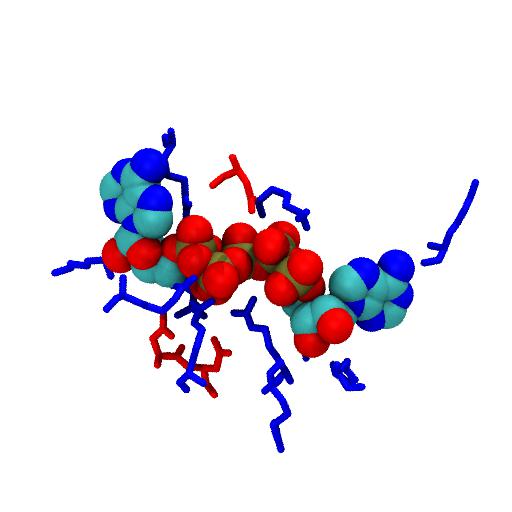
AdK Ligand¶
The AP5 ligand mimics the transition state of the phospho-transfer reaction. Phosphates are negatively charged, hence only basic (positively charged, blue) residues are in contact.