Flexible Gates Generate Occluded Intermediates in the Transport Cycle of LacY
Active secondary transporters are membrane proteins that transport small molecules against an electrochemical gradient by using the energy stored in the transmembrane sodium or proton gradient. Such transporters change their conformation between an outward facing open and and inward facing open conformation as summarized in the alternating access mechanism of transmembrane transport. The major facilitator superfamily (MFS) transporter lactose permease (LacY; TCDB 2.A.1.5.1) is a bacterial symporter that co-transports one lactose molecule together with one proton (H+). Indirect experimental evidence suggested the existence of an occluded transition intermediate of LacY1,2, which would prevent leaking of the proton gradient, but no experimental occluded structure is known. Only experimental crystal structures of inward facing LacY and outward facing homologous proteins have been determined so far.
In the paper Flexible Gates Generate Occluded Intermediates in the Transport Cycle of LacY3 (with Lukas Stelzl and Philip Fowler at the University of Oxford, see also Phil’s blog post about the paper) we employed computational techniques to simulate the hypothesized occluded state of LacY and study the conformational transition between the outward facing open and the inward facing open state.
Occluded state
In order to generate an occluded state of LacY in the apo state (without substrates) we used dynamic importance sampling (DIMS) MD simulations to simulate transitions from the inward facing (known) conformation (PDB id 2V8N) to an outward facing open conformation (modelled by Radestock and Forrest by homology to the FucP outward open structure4, available from the Protein Model Database under accession PM0077183 ), followed by equilibrium MD. Structures were clustered and analyzed in terms of geometry of the translocation pathway and water/proton accessibility.
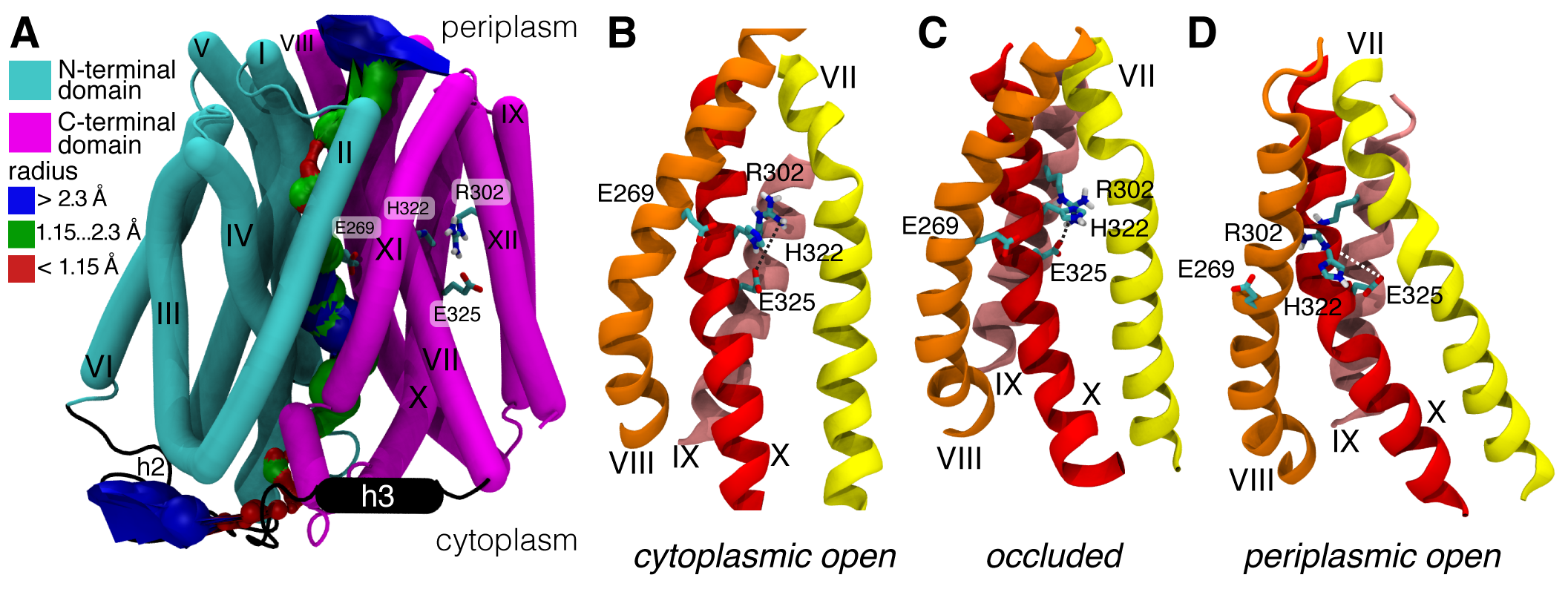
Our model for the occluded state is also available from the Protein Model Database, PM0079313 and in the supplementary information3 as a zipped PDB . Gratifyingly, our model and a recent model by Madej et al5 (which used a completely different approach, namely homology modelling based on the PepTSo structure) agree in the overall conformational changes. In particular, the two domains of LacY will not move as rigid bodies in what was called a “rocker switch” mechanism but instead the extracellular and the intracellular half can each flex on their own and thus form gates that control access to the central binding sites. Our simulations additionally predict the formation of a salt bridge (R302 and E325) that has been hypothesized to form transiently6.
Occluded state of LacY and key residues. A. Occluded state model with labelled helices and highlighted N-terminal (cyan) and C-terminal (magenta) domain. The surface of the translocation pathway as computed by HOLE is shown. Details around the substrate binding site on helices VII, VIII, IX, and X (on the C-terminal domain), including functionally essential residues. B. Cytoplasmic (inward) open (crystal structure). C. occluded (model). A salt bridge between R302 and E325 was formed during the simulation. D. Periplasmic (outward) open (homology model based on FucP).
Comparison to experimental DEER measurements
Our simulations were validated against experimental double-electron electron spin resonance (DEER) measurements2. We employed a novel approach to take the flexibility of the spin labels into account: Phil Fowler’s RotamerConvolveMD package applies Gunnar Jeschke’s MTSL rotamer library7 to a MD trajectory in a post-processing step and then calculates realistic distance distributions of the two spin labels.
RotamerConvolveMD is available as open source under the GNU Public License. It is based on our MDAnalysis Python library.
Comparison of all known MFS transporter structures
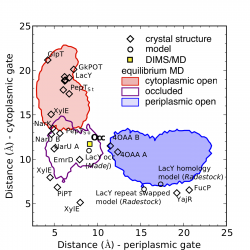
MFS transporter structures classified by gate order parameters. The functional state of MFS transporters can be described by two order parameters: how far the periplasmic and the cytoplasmic gate open up. The plot shows all known MFS transporter structures as diamonds in the "gate space" together with the order parameter regions sampled in our equilibrium MD simulations. The figure is an update on the published figure as it also contains the very recent (2014) partially occluded LacY structure 4OAA (chains A and B)).
We defined a set of two order parameters to classify MFS transporter conformations. The order parameters consist of a distance between residues on TM4 and TM10, the cytoplasmic gate distance, and between residues on TM1 and TM7, the periplasmic gate distance. They describe the extent of opening of the cytoplasmic and periplasmic gate regions. We applied the order parameter analysis to all known MFS transporter structures (including the recent partially occluded structure of LacY8 with PDB ID 4OAA ), structural models including our own model of the apo-occluded state of LacY3 and the model of Madej et al5 together with Radestock’s and Forrests4 model of periplasmic open LacY, and the frames from our equilibrium MD trajectories of LacY.
The plot clearly shows populations of outward-open (i.e. periplasmic open, cytoplasmic closed), inward-open (periplasmic closed, cytoplasmic open), and occluded (both periplasmic and cytoplasmic gate closed) conformations. Occluded states are now recognized as important intermediates the transport cycle of secondary transporters as described by the alternating access model.
Our occluded model (marked by “Occ”) is located in the neighborhood of other MFS transporter structures that have been classified as occluded. The new sugar-bound LacY structure8 appears to be partially occluded and generally retaining an outward-facing appearance. The MD simulations sample conformations (as classified by this choice of order parameters) that contain many of the known structures but notably no transitions between outward-open/occluded/inward-open states were observed on the time scales of the equilibrium MD simulations (100 ns) — sampling of transition pathways required application of the DIMS MD method as described in this work3.
References
1 D. S. Majumdar, I. Smirnova, V. Kasho, E. Nir, X. Kong, S. Weiss, and H. R. Kaback. Single-molecule FRET reveals sugar-induced conformational dynamics in LacY. Proc Natl Acad Sci U S A, 104 (2007) 12640–12645. doi: 10.1073/pnas.0700969104
2 I. Smirnova, V. Kasho, J.-Y. Choe, C. Altenbach, W. L. Hubbell, and H. R. Kaback. Sugar binding induces an outward facing conformation of LacY. Proc Natl Acad Sci U S A, 104 (2007) 16504–9. doi: 10.1073/pnas.0708258104
3 L. S. Stelzl, P. W. Fowler, M. S. Sansom, and O. Beckstein. Flexible gates generate occluded intermediates in the transport cycle of LacY. J Mol Biol 426 (2014), 735–751. doi: 10.1016/j.jmb.2013.10.024 (open access)
4 S. Radestock and L. R. Forrest. The alternating-access mechanism of mfs transporters arises from inverted- topology repeats. J Mol Biol, 407 (2001) 698–715. doi: 10.1016/j.jmb.2011.02.008
5 M.G. Madej, S.N. Soro, H.R. Kaback. Apo-intermediate in the transport cycle of lactose permease (LacY). Proc Natl Acad Sci U S A, 109 (2012), E2970–E2978. doi: 10.1073/pnas.1211183109
6 M. Sahin-Toth, H.R. Kaback. Arg-302 facilitates deprotonation of Glu-325 in the transport mechanism of the lactose permease from Escherichia coli. Proc Natl Acad Sci U S A, 98 (2001), 6068–6073. doi: 10.1073/pnas.111139698
7 Y. Polyhach, E. Bordignon, and G. Jeschke. Rotamer libraries of spin labelled cysteines for protein studies. Phys. Chem. Chem. Phys., 13 (2011) 2356-2366. doi: 10.1039/C0CP01865A
8 H. Kumar, V. Kasho, I. Smirnova, J.S. Finer-Moore, H.R. Kaback, and R.M. Stroud. Structure of sugar-bound LacY. Proc Natl Acad Sci U S A, 111 (2014), 1784–1788. doi: 10.1073/pnas.1324141111


Discuss: “Flexible Gates Generate Occluded Intermediates in the Transport Cycle of LacY”