Structure and function of the mammalian sodium/proton exchanger NHE9
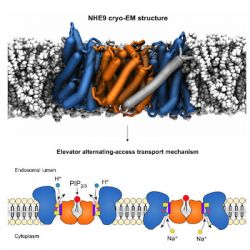
NHE9 is a sodium/proton exchanger in mammals, part of a large family of secondary active transporters. Although a number of structures of prokaryotic sodium/proton antiporters had been solved in the past, there was no mammalian structure available. Our collaborators (David Drew at the University of Stockholm) solved the structure of NHE9 (SLC9A9) from horse with cryo electron-microscopy to 3.2 Å resolution in an inward facing conformation1. The first structure of a mammalian CPA1 (cation-proton antiporter 1) shows that the overall architecture is very similar to the prokaryotic structures that we already know. Functional measurements, structural bioinformatics, and molecular dynamics simulations all point to common components of the transport mechanism such as the sodium and proton binding sites and the large elevator conformational transition between inward facing and outward facing conformations. Thus, lessons learned from the prokaryotic structures (such as the molecular basis of ion translocation in the prokaryotic NapA sodium/proton antiporter) will likely also be applicable to the mammalian and in particular human homologs.
The paper made the cover of EMBO Journal's December issue.
Cover of the EMBO J Volume 39, Issue 24 (2020) showing the NHE9 membrane protein from MD simulations in a lipid membrane. The image was created by Ricky Sexton using VMD.
References
- a I. Winkelmann, R. Matsuoka, P. F. Meier, D. Shutin, C. Zhang, L. Orellana, R. Sexton, M. Landreh, C. V. Robinson, O. Beckstein, and D. Drew, “Structure and elevator mechanism of the mammalian sodium/proton exchanger NHE9,” The EMBO Journal, vol. 39, no. 24, p. e105908, 2020. doi: 10.15252/embj.2020105908




Discuss: “Structure and function of the mammalian sodium/proton exchanger NHE9”