
Molecular dynamics simulations
Molecular dynamics (MD) is a computational method to compute the trajectories of a large number of particles that interact with each other. The most common approach is to simulate individual atoms but coarse grained approaches are also popular, which lump a number of atoms in pseudo particles. For atomistic simulations, current (2012) state of the art (classical) simulation approaches allow one to treat systems ranging from thousands to ~1–5 million particles.
In principle, the many-body Schrödinger equation describes the dynamics of systems that are studied by biologists. It is, however, too difficult to solve for more than a few hundred atoms and therefore we need to approximate the quantum mechanical solution. Classical MD approximates interactions between atoms by classical forces such as harmonic springs for chemical bonds or the electrostatic Coulomb interactions between electrons with Coulomb’s law between fixed partial charges on atoms.
By using Newton’s second law, F = ma with a = d2x/dt2, we can integrate the equations of motion for all atoms and obtain a trajectory x(t) of all particles in the system. In practice, the integration is carried out for small timesteps Δt, which are much shorter than the fastest motions in the system (for biological systems, timesteps of 1–2 fs are typically used). The trajectory is analyzed using statistical mechanical approaches but also computer graphics. The simulation provides molecular detail at each timestep – one can get the positions (and velocities) of every individual atom – but often the challenge is to use this detailed knowledge to calculate experimental observables.
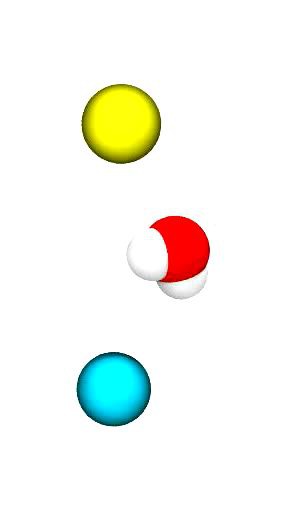
The movie (click on the image) shows a short, very simple trajectory from a MD simulation of a single water molecule (oxygen in red, hydrogens in white) between two ions: a sodium ion (Na+ in cyan) and a chloride ion (Cl– in yellow). The ions are harmonically restrained to their initial positions for this simulation (otherwise they would fly into each other) but the water molecule is free to move under the influence of the ions.



Discuss: “Molecular dynamics simulations”