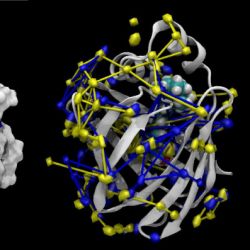
Protein hydration
Proteins interact with the surrounding water, and water molecules influence the binding of ligands and drugs to proteins profoundly. Sites of preferential water occupancy (hydration sites) can be inferred from high resolution crystal structures or molecular dynamics simulations (as shown here). Water molecules move between hydration sites on the exterior and the interior and also the bulk (not shown) and these movemnts can be represented as a network. Such a coarse-grained analysis of water dynamics was carried out with our HOP analysis software. The protein shown here is CRBPII, a retinol binding protein. The hydration network between the apo protein (blue, without a ligand) and holo (yellow, with a retinol molecule bound) shows a number of conserved hydration sites, especially on the inside.


